Viz.ai Receives First and Only FDA 510(k) Clearance for Subdural Measurements
Viz.ai Receives First and Only FDA 510(k) Clearance for Subdural Measurements
New AI algorithm enables precise and reliable labeling, visualization and quantification of subdural hemorrhage to make accurate and timely treatment decisions
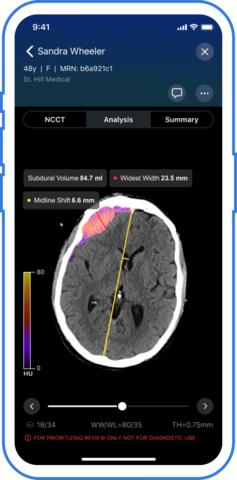
SAN FRANCISCO--(BUSINESS WIRE)--Viz.ai, the leader in AI-powered disease detection and intelligent care coordination, today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for Viz Subdural Plus, the first and only comprehensive solution for quantifying the size of collections like subdural hemorrhages (SDH) in the subdural space on non-contrast computed tomography (NCCT) images. The Viz Subdural Plus module is designed to support clinical-decision making by automatically labeling subdural collections and reporting measurements, including volume, thickness, and midline shift.
“Viz Subdural Plus introduces a new level of precision in diagnosing and monitoring subdural hemorrhage,” said David J. Altschul, MD, Division Chief Cerebrovascular Neurosurgery at Montefiore Health System. “Having automated volume and max thickness measurements at our fingertips allows us to make faster, more informed treatment decisions—especially critical in managing elderly patients or those on anticoagulants. As we increasingly turn to minimally invasive options like MMA embolization to reduce recurrence, tools like Viz Subdural Plus are essential to guiding timely and effective treatment.”
Chronic subdural hematoma (SDH) is projected to be the most common cranial neurosurgical condition in adults with projections suggesting approximately 60,000 new cases diagnosed annually in the United States by 20301 due to the aging population and more widespread use of anticoagulant and antiplatelet medications. Accurate quantification of subdural collections is essential for evaluating severity, monitoring progression, and informing timely treatment– especially for patients being considered for MMA embolization. Viz Subdural Plus supports clinicians in streamlining subdural collection analysis by automating what has traditionally been a manual and time-consuming measurement process. The software is available as part of the Viz.ai One platform, which is currently deployed across 1,800 hospitals and health systems.
"With an aging population, the incidence of chronic subdural hematomas is rising, and so is the need for intelligent, automated tools to assess volume when deciding on the appropriate intervention, such as MMA embolization," said Justin Ryea, Senior Director of Product Management at Viz.ai. "Viz Subdural Plus, along with intracerebral hemorrhage measurements, exemplify how we're expanding our capabilities in our market-leading Viz Neuro Suite to address high-impact conditions, reduce variability in care, and drive better outcomes at scale."
To learn more about Viz Subdural Plus, please visit viz.ai/hemorrhage.
1. Neifert, S.N., Chaman, E.K., Hardigan, T., et.al. (2020). Increases in subdural hematoma with an aging population- the future of cerebrovascular disease. World Neurosurgery, 141, 166-174.
About Viz.ai, Inc.
Viz.ai is the pioneer in the use of AI algorithms and machine learning to increase the speed of diagnosis and care across 1,800 hospitals and health systems in the U.S. and Europe. The AI-powered Viz.ai One® is an intelligent care coordination solution that identifies more patients with a suspected disease, informs critical decisions at the point of care, and optimizes care pathways and helps improve outcomes. Backed by real-world clinical evidence, Viz.ai One delivers significant value to patients, providers, and pharmaceutical and medical device companies. For more information visit Viz.ai.
Contacts
Media Contacts
Carolyn Jones
carolyn.jones@viz.ai
Daniel Yunger
daniel.yunger@kekstcnc.com