RevBio Initiates its Pivotal Clinical Trial in Europe for its Dental Implant Stabilization Product
RevBio Initiates its Pivotal Clinical Trial in Europe for its Dental Implant Stabilization Product
The Company Received Approval in Multiple European Countries to Initiate this Key Study Necessary for Commercial Product Approval
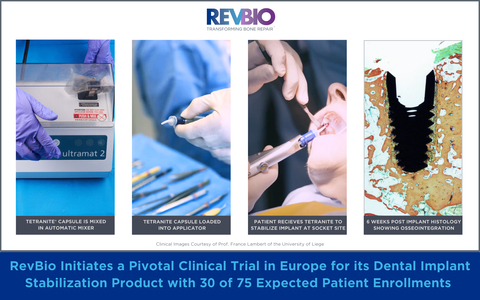
LOWELL, Mass.--(BUSINESS WIRE)--RevBio, Inc., announced that it has received regulatory and ethics committee approvals in multiple European countries to conduct its pivotal clinical trial for its dental implant stabilization product. The successful completion of this pivotal clinical trial will result in the CE marking approval for the product, which will allow the company to begin commercial sales in Europe. As of the date of this press release, the company has already enrolled 30 of an expected 75 patients in this clinical trial.
"The approval to conduct this pivotal clinical trial and the successful enrollment of the first 30 patients is a watershed moment for RevBio," said Alan Pollack, DDS, RevBio’s Senior Director of Dental Clinical Operations.
Share
“The results from this ongoing international multicenter study are exceeding expectations, showcasing remarkable outcomes. We are profoundly optimistic and incredibly enthusiastic about what the future holds,” said Prof. Dr. med. Dent., Patrick Schmidlin, head of the Division of Periodontology at the University of Zurich, who serves as one of the investigators in RevBio’s pivotal clinical trial. “This breakthrough material promises to open a new chapter in dental care, redefining what is possible in regenerative dentistry.”
RevBio received regulatory and ethics committee approvals for five clinical sites consisting of one in Switzerland, two in Belgium, one in Spain, and one in the United Kingdom. Each site is expected to enroll between 12-25 patients for a total of approximately 75 patients. The investigators who are approved to enroll patients in this clinical trial are Prof. Dr. med. Dent., PhD, Patrick Schmidlin, head of the Department of Periodontology at the University of Zurich, Ana Castro, BDS MSc PhD, Clinical Head Department Periodontology at the Catholic University of Leuven, France Lambert, DDS, PhD, Professor and Head of Periodontology, Oral Surgery, and Implant Surgery at the University of Liege, Arturo Llobell, DDS, MS, a periodontist in private practice in Valencia, Spain, and Azim Malik, BDS, MFDS RCSEd, DipPCD RCSI, DClinDent, MPerio RCSEd, a periodontist and implant specialist in private practice in London, United Kingdom.
When teeth are extracted due to damage from traumatic injuries, tooth decay, or gum disease, the current standard of care consists of multiple staged surgical procedures to restore a patient’s dentition with prosthetic crowns supported by dental implants. Frequently, extraction sites are too large for dental implants to achieve primary stability through conventional mechanical engagement. Instead, patients must undergo a costly, complex, and lengthy process including a preliminary bone grafting surgery before receiving a dental implant. The use of TETRANITE to stabilize an unstable implant will allow for the immediate placement of dental implants which otherwise could not be placed until the initial bone graft has healed to form new bone. As a result, the TETRANITE® biomaterial will help reduce the duration and complexity of these dental implant procedures, lessen patient pain and recovery time, and reduce the overall cost of care thereby providing greater patient access for the treatment of tooth loss.
"The approval to conduct this pivotal clinical trial and the successful enrollment of the first 30 patients is a watershed moment for RevBio," said Alan Pollack, DDS, RevBio’s Senior Director of Dental Clinical Operations. “This clinical trial has the potential to significantly accelerate the timeframe when this promising bone adhesive technology can be used in the field of dentistry.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of TETRANITE®, a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial. The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio's TETRANITE technology is not yet approved for commercial use.
Contacts
Michael Tiedemann
mtiedemann@revbio.com