Aptatek BioSciences, Inc. Announces Bridge Financing to Support Advancement of the PheCheck™ Aptamer-based In-home Monitoring Platform
Aptatek BioSciences, Inc. Announces Bridge Financing to Support Advancement of the PheCheck™ Aptamer-based In-home Monitoring Platform
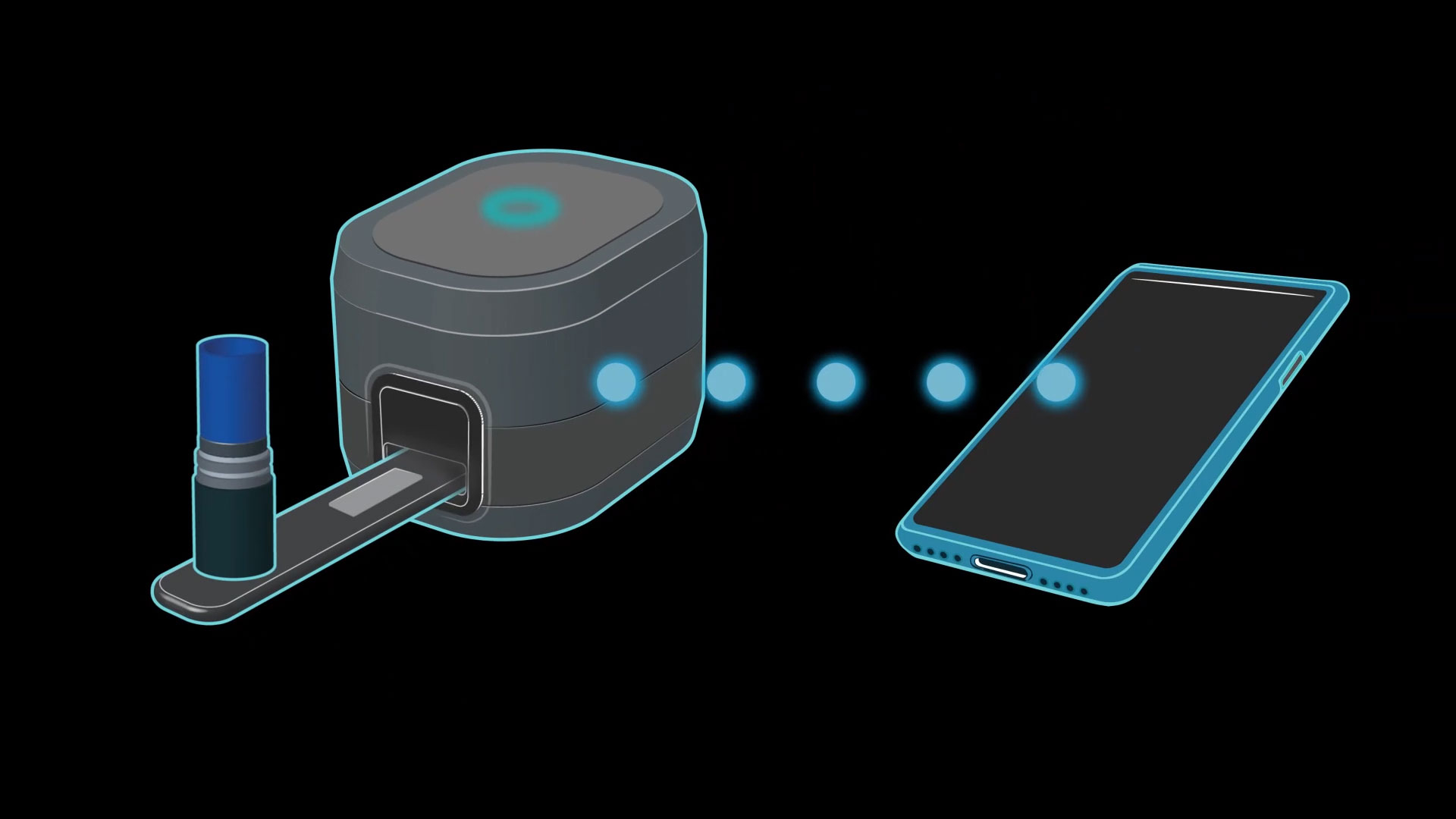
PRINCETON, N.J.--(BUSINESS WIRE)--Aptatek BioSciences, Inc., a pioneer in developing innovative in-home monitoring solutions for patients managing chronic diseases, today announced it has received bridge financing from Canterbury Scientific, Ltd, a current strategic investor. This latest funding tranche follows the successful advancement of Aptatek’s PheCheck™ platform, an FDA breakthrough diagnostic device designed for at-home monitoring of phenylalanine levels in patients with phenylketonuria (PKU).
Transforming chronic disease management through home monitoring solutions
Share
The new financing supports Aptatek’s ongoing clinical development and accelerates its path toward commercialization of its DNA aptamer-based home monitor for PKU, a genetic metabolic disorder, requiring diligent monitoring of phenylalanine levels to prevent serious health complications. As with diabetes management, maintaining optimal phenylalanine levels through diet and medication is essential for patient health and quality of life.
“This further funding from Canterbury Scientific strengthens our ability to bring our groundbreaking monitoring solution to PKU patients,” stated Dr. Michael Boyce-Jacino, CEO of Aptatek. “We deeply value Canterbury’s continued confidence and strategic partnership as we move closer to commercialization.”
Clive Seymour, CEO of Canterbury Scientific, a leading global OEM supplier of IVD controls, added, “Aptatek is at the forefront of revolutionizing home health management. We are excited to support the commercialization of their innovative and patient-centric approach to chronic disease monitoring, offering patients greater control over their health outcomes and daily lives.”
Founded as a spin-out from Columbia University, Aptatek BioSciences, Inc. leverages advanced aptamer technology to create simple, reliable, quantitative, point-of-care diagnostics that empower patients with chronic conditions to manage their health effectively from home.
Contacts
Michael Boyce-Jacino, CEO, Aptatek BioSciences, Inc.
info@aptatek.com, (609) 474-0716